Como resultados, o estudo demonstrou que as concentrações mínimas de pertuzumabe e trastuzumabe foram não-inferiores nas pacientes tratadas com PHESGO® em comparação àquelas que receberam a administração da terapia endovenosa convencional. No tocante à eficácia, a taxa de resposta completa foi 59,7% no braço que recebeu PHESGO® e.. Outros efeitos colaterais incluem diarréia, alterações gastrointestinais como náuseas, dores no corpo e muito raramente pode comprometer a função do coração. É muito importante manter um acompanhamento constante com a equipe médica durante o tratamento com PERTUZUMABE. Isto deve ser planejado e bem discutido pois algumas pacientes com.
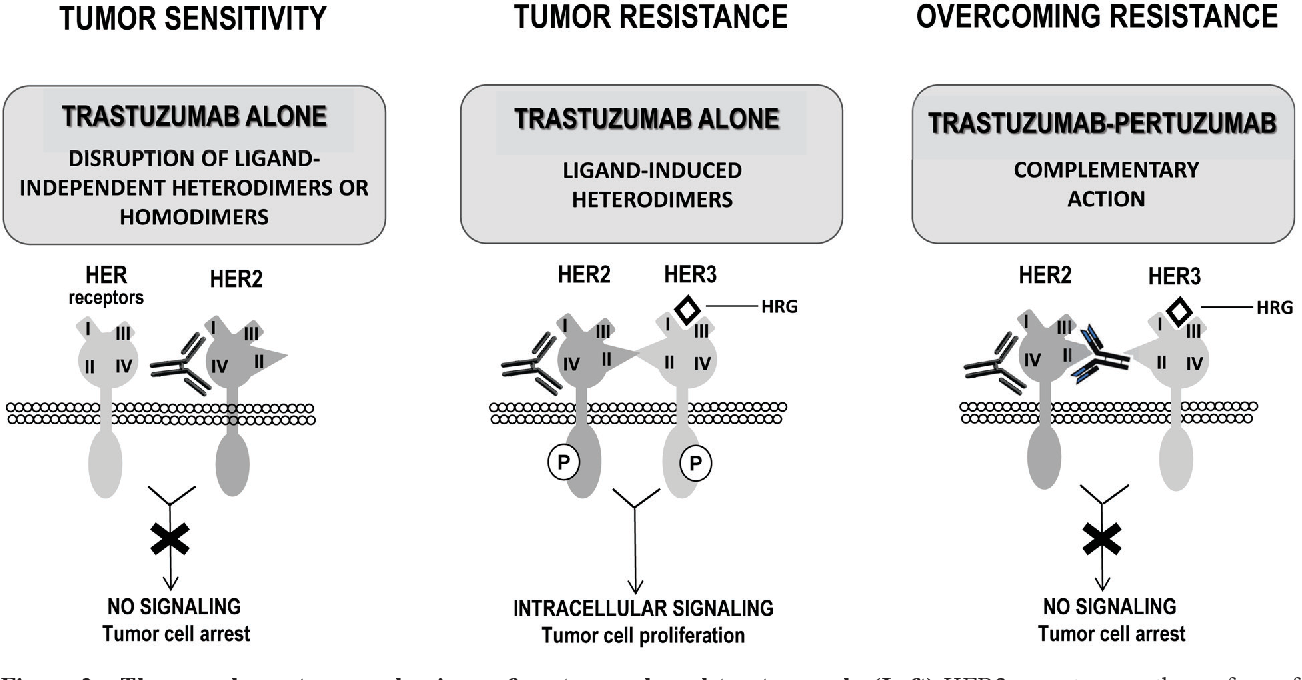
Figure 2 from Pertuzumab and trastuzumab the rationale way to synergy. Semantic Scholar
Trastuzumabe e pertuzumabe poderão ser incorporados ao SUS

Trastuzumab schematic structure. The structure of HER2 ectodomain in... Download Scientific

HER2 inhibitors such as trastuzumab and pertuzumab are antibodies that... Download Scientific
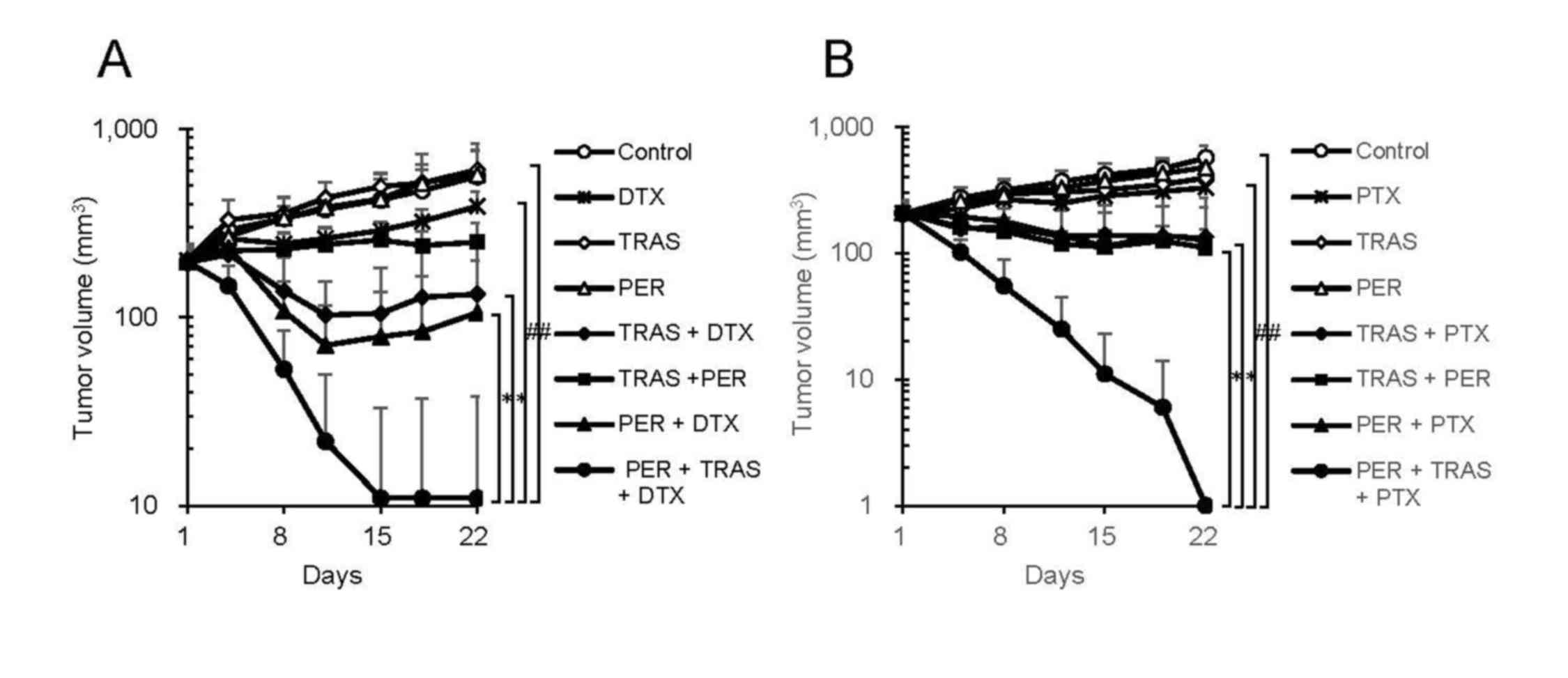
Mode of action of pertuzumab in combination with trastuzumab plus docetaxel therapy in a HER2
Uso de trastuzumabe deruxtecana em pacientes com CPNPC HER2 mutado Oncologia Brasil

Pertuzumab 10 2014 Heftarchiv AMT

Phesgo Rx Pertuzumab Trastuzumab Injection Roche
HER2 dual inhibition by trastuzumab and pertuzumab and intracellular... Download Scientific
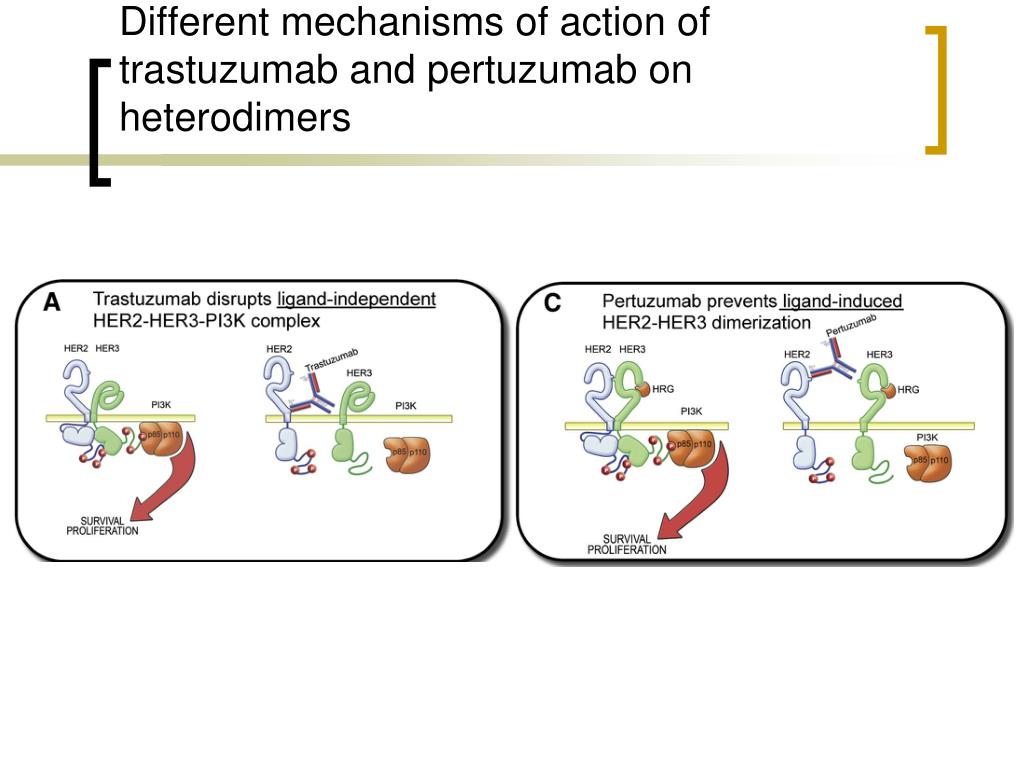
PPT Pertuzumab e TrastuzumabDM1. PowerPoint Presentation, free download ID1450308
![[PDF] Resistance to Trastuzumab in Breast Cancer Semantic Scholar [PDF] Resistance to Trastuzumab in Breast Cancer Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0b7c21f16969d3bdb4df0d11df47495b5084dbd5/8-Figure5-1.png)
[PDF] Resistance to Trastuzumab in Breast Cancer Semantic Scholar
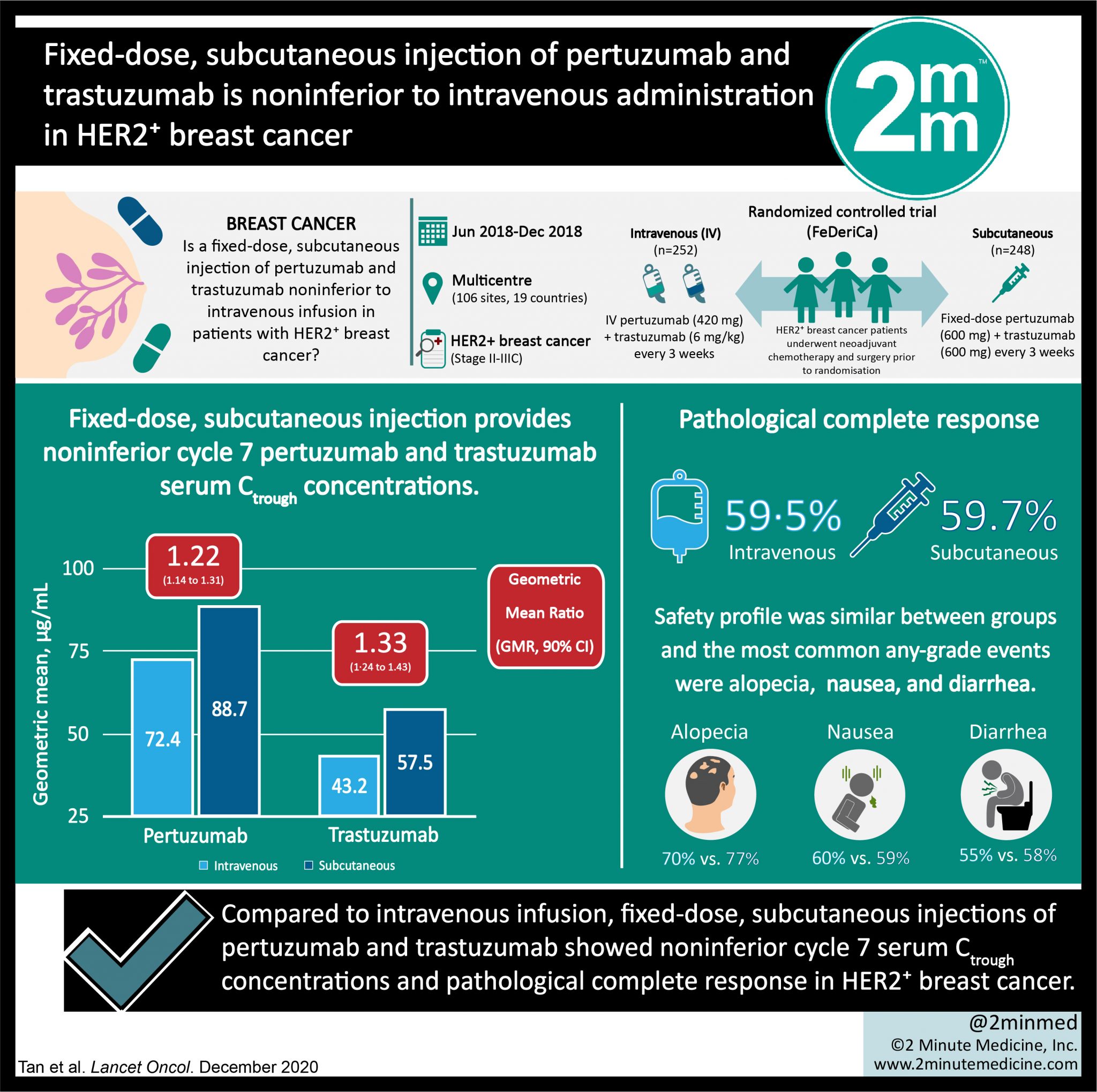
VisualAbstract Fixeddose, subcutaneous injection of pertuzumab and trastuzumab is noninferior
Nueva presentación de 'Herzuma' (trastuzumab) para oncología DiarioMedico
Trastuzumab Structure
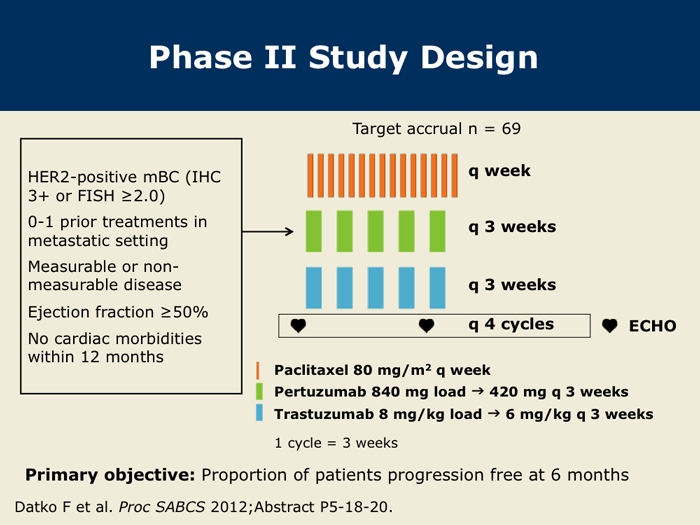
Phase II Study of Pertuzumab, Trastuzumab and Weekly Paclitaxel in HER2Overexpressing

Overall structure of HER2trastuzumabpertuzumab and comparison with... Download Scientific
Bispecific trastuzumab/pertuzumab plant biosimilars and SPR analysis of... Download Scientific

Figure 1 Structure of trastuzumab emtansine and mechanisms of action. Notes On binding of

Pertuzumab a cosa serve, come funziona, effetti collaterali
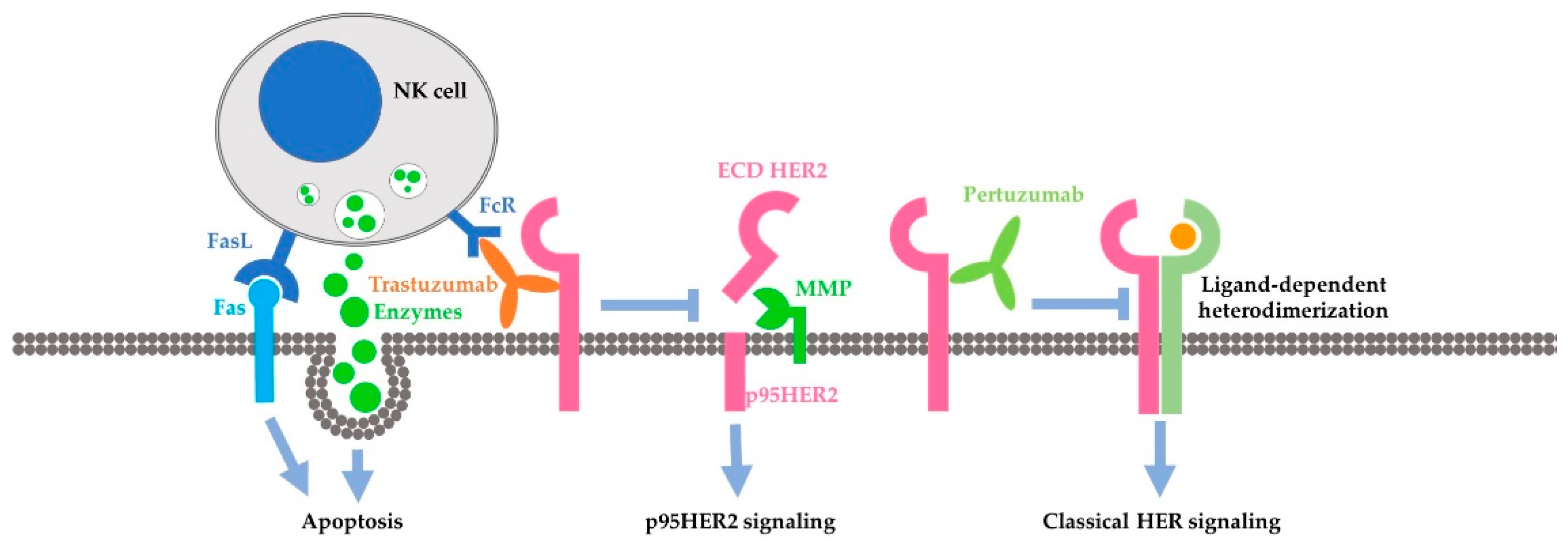
Cancers Free FullText Mechanisms Underlying the Action and Synergism of Trastuzumab and
a eficácia/efetividade, segurança, custo-efetividade e impacto orçamentário do trastuzumabe entansina em monoterapia para tratamento de pacientes com câncer de mama HER2-positivo metastático ou localmente avançado irressecável, que tenham recebido tratamento prévio com trastuzumabe e um taxano, na perspectiva do SUS. 2.. In the trial population, 63% of the patients who were randomly assigned to receive pertuzumab (2400 patients) or placebo (2405 patients) had node-positive disease and 36% had hormone-receptor.